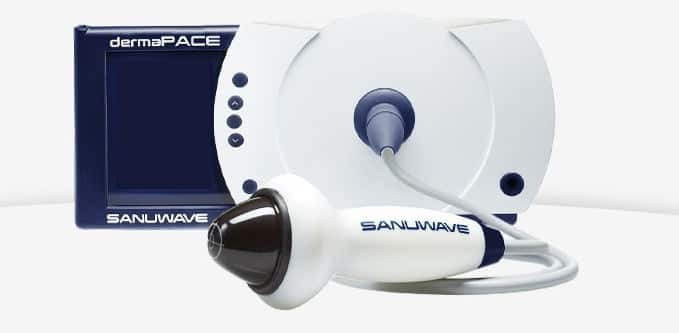
Shock waves aren’t just for Overwatch, FDA (An agency within the U.S. Department of Health and Human Services) has approved a Sanuwave for its dermaPACE (Pulsed Acoustic Cellular Expression) System, which treats chronic and acute conditions of the skin and subcutaneous tissue.
ALSO READ: FCC Approves Energous WattUp Power-At-A-Distance Charging System!
First Shock Wave Device; dermaPACE!
The approval of dermaPACE System is specifically to help heal foot ulcers in diabetic patients, where the damaged blood vessels and nerves can lead to reduced circulation, infection or sometimes amputation.
So, what dermaPACE does is that it stimulates the wound and promotes healing. Much like any other FDA approved devices, the device also went through the de novo premarket review pathway, designed specifically to get the technology out on the market.
For the latest tech news, follow TechDipper on Twitter, Facebook, Google+, Instagram and subscribe to our YouTube channel.